A diamond is an organised assembly of carbon atoms, transparent to visible light.
There are many natural diamond hues, often sought after in jewellery (Figure 1a-d), which are due to impurities and defects in the carbon structure that absorb light.
The nitrogen-vacancy center
Amongst these numerous coloured defects (more than 500 are listed [1]), the Nitrogen-Vacancy center or NV has unique properties.
An NV center (see Figure 1d) consists of a nitrogen atom (N) that has been substituted for a carbon atom, and a neighbouring atom site that is left vacant (V).
The NV center can be thought of as an artificial molecule trapped in a solid diamond. It is therefore easy to manipulate, and extremely small (a few Angstroms).
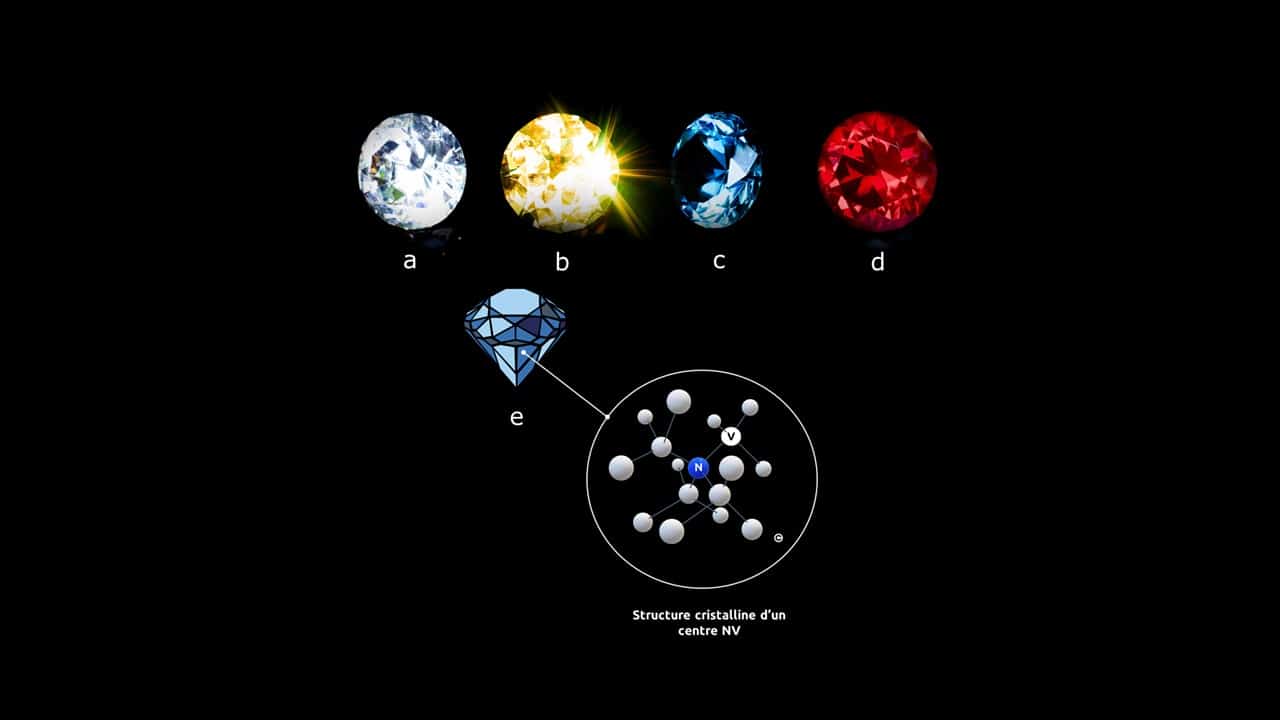
Figure 1 – Centre NV – (a-d) – exemples de diamants colorés – (e) Schéma d’une maille de diamant contenant un centre NV. Un atome de carbone est remplacé par un atome d’azote (N), et un atome voisin est manquant (lacune- V)
The propertries of a NV center
The NV center is a perfectly stable single photon source.
When illuminated by green light, the NV center re-emits red photoluminescent light.
Unlike many pigments or molecules, the NV center never disappears. This makes it the luminescent marker of choice for biology [2].
Furthermore, a single NV center emits single photons, one after the other. This property makes the NV center an ideal candidate as a light source for quantum optics, and it has been used in fundamental tests such as the Wheeler delayed choice experiment [3, 4].
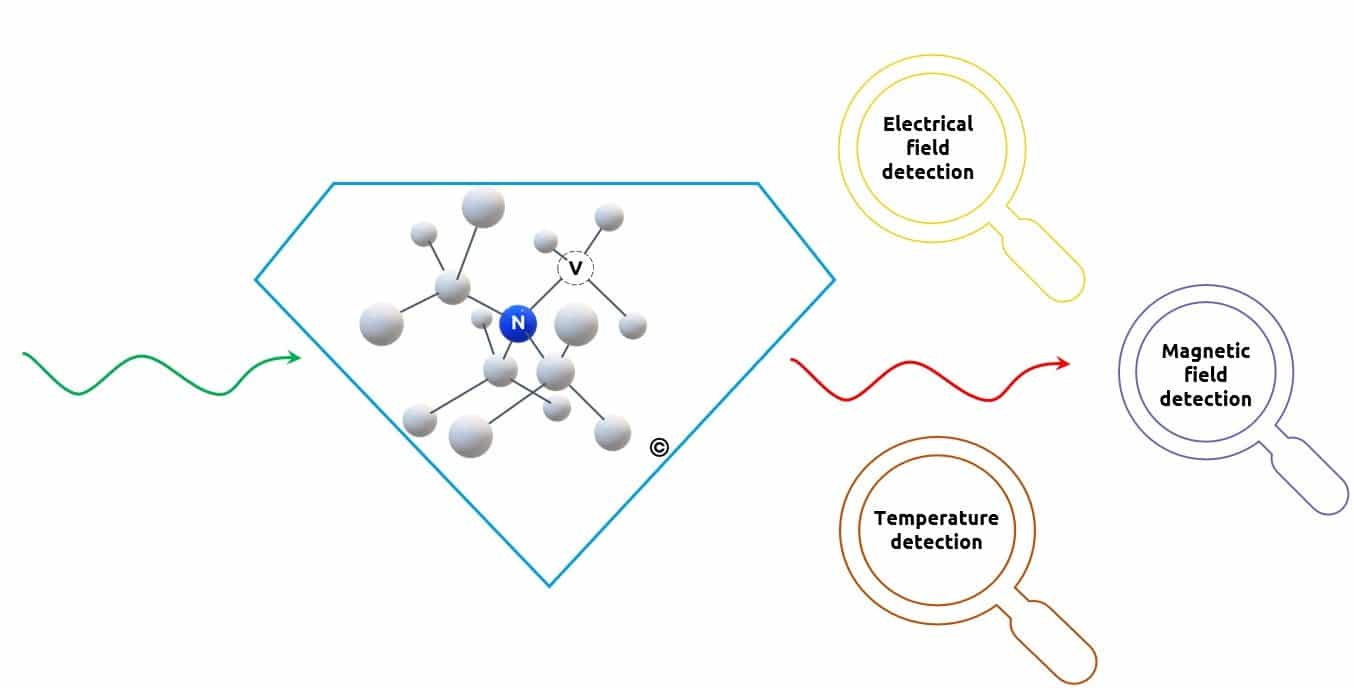
In addition to its optical properties, the NV center has unique spin properties.
A spin is the quantum-scale equivalent of a tiny magnet, like the needle of a compass. When illuminated with green light, the spin of the NV center can be prepared in a desired state.
On the other hand, one can directly read the spin state of the NV center by looking at the amount of red light it emits.
By preparing the spin of the NV center in a known state, we can study its interaction with the environment. This is done by optically measuring the evolution of its state under different conditions of temperature, magnetic field or electric field.
In this way, the NV center is used as a quantum sensor, which can measure various quantities very precisely and with atomic resolution.
The production of NV center diamonds
In order to use the NV center, it is important to be able to control its production.
The NV center occurs naturally in most diamonds due to the abundance of nitrogen in the air.
However, in order to have sufficient densities of these centers, or to control their position, the diamond must be doped with nitrogen.
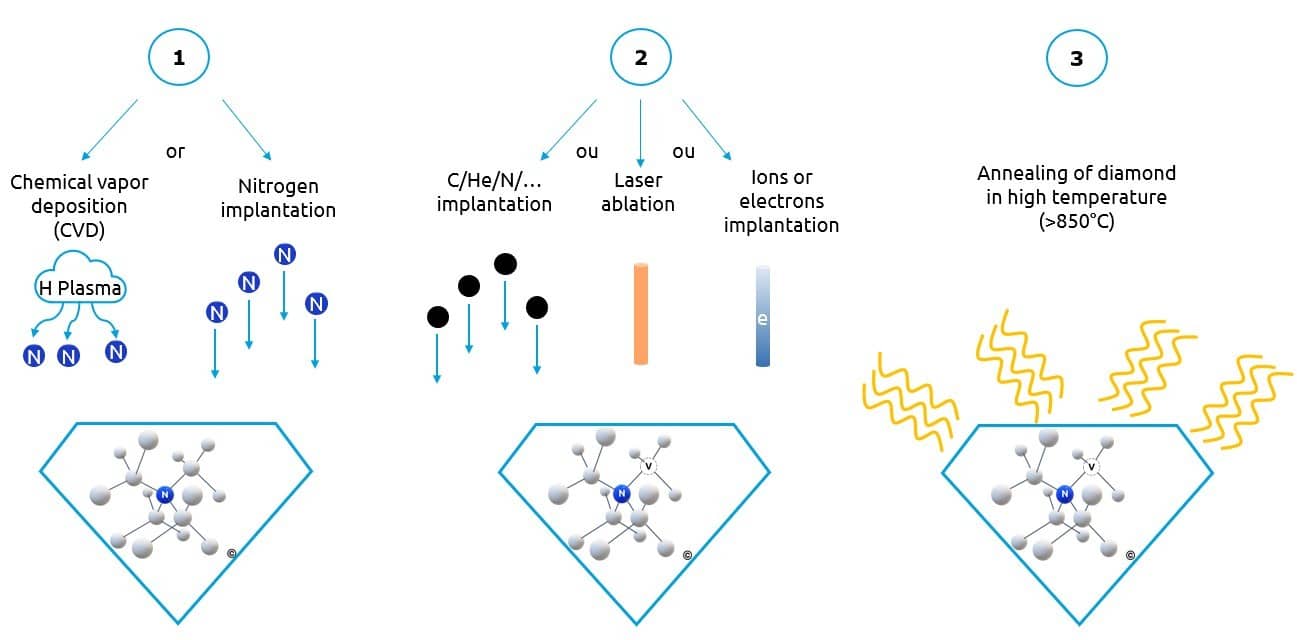
This can be done either by adding nitrogen during the creation of a diamond by chemical vapour deposition (CVD) or by implanting a diamond with nitrogen to modify it.
In both cases, it is then necessary to create gaps in the diamond, i.e., remove some carbon atoms. This can be done by laser ablation, or by implanting the diamond with ion or electron beams.
The final step is annealing the diamond, at high temperature (> 850°C), to rearrange the diamond matrix and promote the migration of vacancies to the nitrogen atoms and obtain NV centers.
In this way, it is possible to have diamonds that are highly doped with NV centers, and to control their position in the diamond [5], in order to manufacture them according to the targeted applications.
References
[1] Zaitsev A. M., Optical Properties of Diamond (2001)
[2] Yuen Yung Hui et al 2010 J. Phys. D: Appl. Phys. 43 374021
[2] Jacques, Vincent; Wu, E; Grosshans, Frédéric; Treussart, François; Grangier, Philippe; Aspect, Alain; Roch, Jean-François (2007). “Experimental Realization of Wheeler’s Delayed-Choice Gedanken Experiment”. Science. 315 (5814): 966–8.
[3] Wikipédia
[5] Smith, Jason M., Meynell, Simon A., Bleszynski Jayich, Ania C. and Meijer, Jan. “Colour centre generation in diamond for quantum technologies” Nanophotonics, vol. 8, no. 11, 2019, pp. 1889-1906.
J Achard et al 2020 J. Phys. D: Appl. Phys. 53 313001, https://iopscience.iop.org/article/10.1088/1361-6463/ab81d1