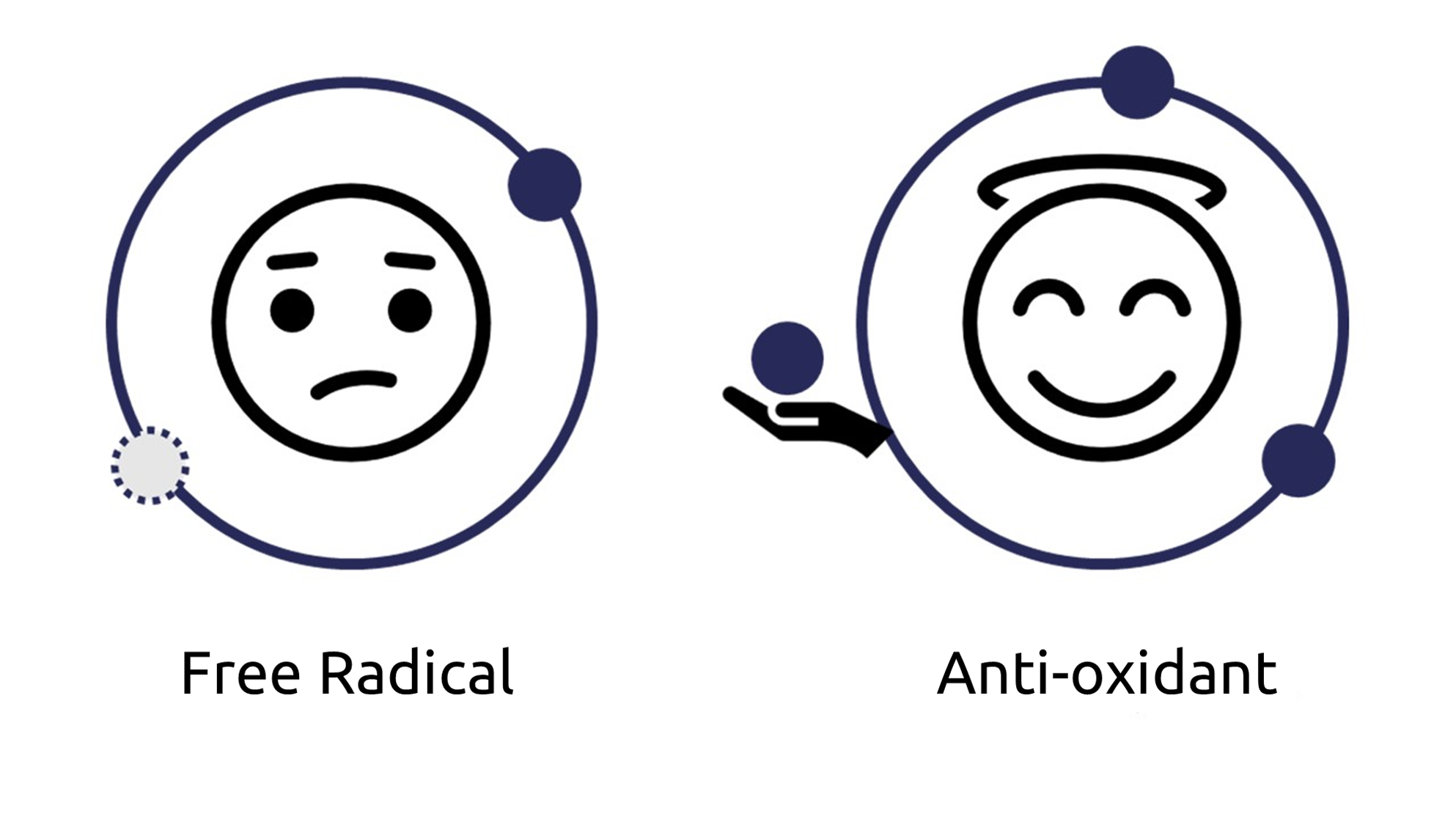
Free radicals are molecules with a free electron, which makes them highly reactive. They are naturally present in various systems of everyday life such as plastics, oils and even the cells of our body. When they are present in a large quantity, they can damage their environment.
In synthetic materials such as plastics or lubricants, they are created by degradation mechanisms (by light, heat, humidity or other) and aggravate this degradation.
In cells, they are generated by healthy and natural mechanisms but can also be involved in pathologies such as infections or cancers.
Anti-oxidants
An antioxidant is a molecule capable of donating or capturing an electron while remaining stable. In this way, it inhibits or delays undesirable oxidation reactions. The ancient Egyptians used various antioxidant substances for mummification, such as beeswax and onions [1]. Indeed, antioxidants prevent the formation of reactive oxygen species (ROS), thus limiting oxidative stress and, consequently, the deterioration of a product or a body. It is therefore interesting to measure the antioxidant capacity of biological samples.
References
[1] R.Apak, Current Issues in Antioxidant Measurement, Journal of Agricultural and Food Chemistry, 2019.
S. Balasaheb Nimse et D. Pal, « Free radicals, natural antioxidants, and their reaction
mechanisms », RSC Advances, vol. 5, no 35, p. 27986‑28006, 2015, doi:10.1039/C4RA13315C.